Technology
Our enterprise-ready clinical trial data platform supports the entire clinical trial process: from wearable data collection to deep scientific analysis that influences decisions. We provide a robust infrastructure for high velocity, high volume patient data capture, storage, and analysis, taking into account the complexities of synchronizing multiple wearable devices, sensors, and data streams.
Our enterprise-ready clinical trial data platform supports the entire clinical trial process: from wearable data collection to deep scientific analysis that influences decisions. We provide a robust infrastructure for high velocity, high volume patient data capture, storage, and analysis, taking into account the complexities of synchronizing multiple wearable devices, sensors, and data streams.
Research-ready infrastructure for real world data.
Secure, Reliable Data Engineering
Flexible Deployment
Sustainable Data Quality
Secure by Design
Our stack of applications are device agnostic and ideal for early-stage trials, registry work, observational studies, and post-market research.
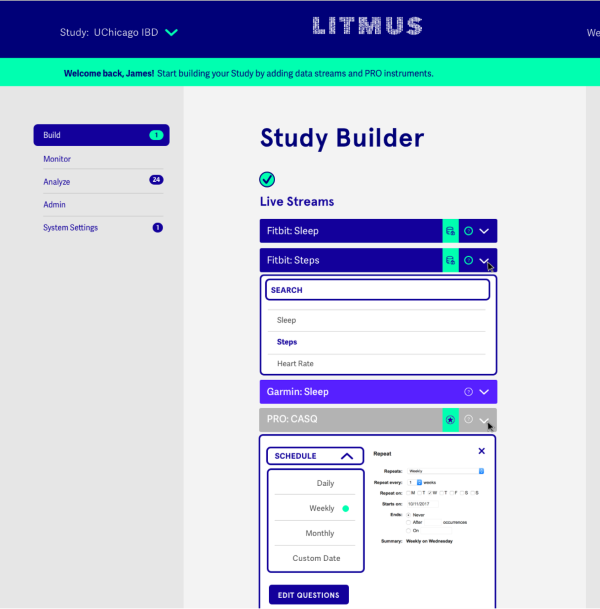
Trial Builder
Identify, input, and measure the unique components of your clinical trials
Our modular system automates the process of establishing electronic data capture systems for studies. The platform supports more than 200 data sources that describe patients’ behavior and environment. We also draw from a library of validated surveys and can integrate with additional instruments.
- Create a study or data collection tool using a simple interface.
- Select one or more data streams or define and add your own.
- Gather from wearables, non-wearable sensors, smartphones, other devices or surveys, and ePROs.
- Import into other systems for downstream analysis and interpretation.
Trial Companion App
A single application that spans multiple clinical trials, reducing time and costs
The Litmus Trial Companion mobile app accurately gathers external wearable device and phone data, sending information back to our platform in real-time. The app also handles ePro survey responses, which can be collected at regular intervals or triggered by external events. A low-friction, easy data collection experience for your patients means more timely and accurate data for your clinical trial. Litmus Trial Companion is available for iOS and Android phones.
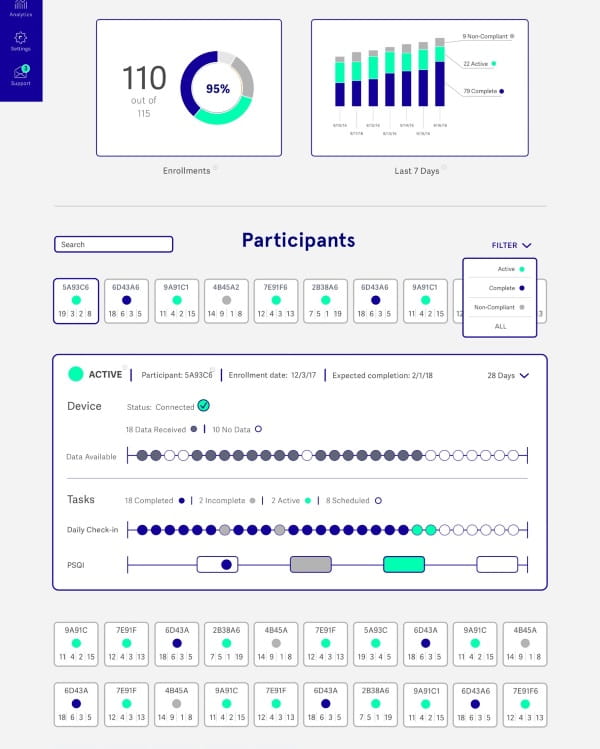
Study Hub
Identify trends and track outcomes across variables with one, unified dashboard
The Litmus Health Study Hub displays real-time data that indicates your study’s progress. See patient population trends at-a-glance and then easily drill down to monitor individual participants’ data. Ensure participant compliance and understand the overall progression of a study.
- Understand enrollment and adherence at-a-glance.
- Program customizable features, such as push notifications and text messages.
- Let Litmus Health respectfully remind patients to take the desired action of your trial.
- Download whole or sliced raw, well-shaped JSON data.
- Configure persistent, faceted searches to closely monitor particular trends or cohorts.

Litmus ML
Litmus Health is integrated with the open, validated community tools you already use every day
Litmus ML modules help you quickly align and interpolate time series data, integrate multiple orthogonal data sources, and look for correlations between behavior, environment, and patient outcomes.
- Combine heterogeneous data streams into one normalized corpus for analysis.
- Get instant access to data in your notebook of choice; there’s no data wrangling and no pipeline involved.
- Proprietary modeling of errors normalizes device variance and reduces uncertainty across clinical trial data.
